One and a half billion years ago, more or less the whole Earth was covered by a single warm ocean. The air outside the ocean was filled with oxygen, a violent poison that uncompromisingly killed most living organisms that were exposed to it.
But a few species of bacteria hacked this killing system and learned to use the oxygen to produce energy. They learned to produce triphosphorylated adenosine with two macroergic bonds, or in common speech, ATP.
One of these bacterial species eventually incorporated itself into another type of cell, and over the next billion years, the cells thus combined continued to evolve, enjoying their synergy with each other, until they eventually created all the animals, and ultimately humans (which is just a strange type of animal that human-centrists don’t consider to be an animal).
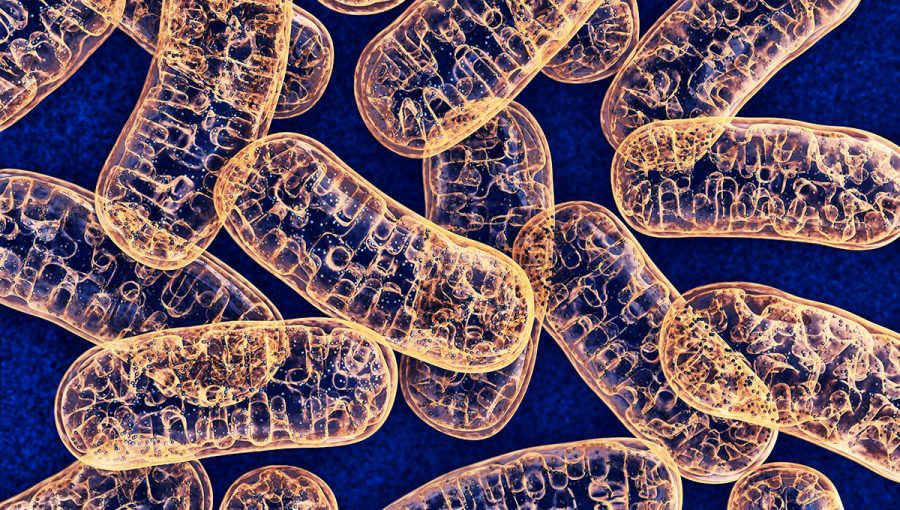
These oxygen-hacking bacteria still reside inside our cells, where they produce ATP, the energy that all our cells need to live.
Today, however, we don’t call them “oxygen-hacking bacteria”, but “mitochondria”.
We’ve known about the existence of ATP for about a hundred years, and we still have a lot to learn about it. We know that ATP is essential for our cellular recharging and we could not survive without it (for example, we can survive for three days without water, but without ATP we would die in seconds).
The energy stored in ATP is released the moment the body needs to use it as fuel. At that point ATP is broken down into two products — adenosine diphosphate and phosphate. And anyone who knows the basics of mathematics and internationalisms must have figured out that since ATP is an adenosine triphosphate, it contains three phosphate bonds. When one of the bonds breaks off to form an adenosine diphosphate and one separate phosphate, energy is released to power us. And so this quadrillion tiny bacteria incorporated into cells ultimately controls what we dare to call “us”.
When the process of ATP breakdown is complete, cool stuff happens. Our body attaches the phosphate molecule back to ADP, thus creating ATP again, which can then be split back into ADP and P to produce more energy. So the mitochondria produce energy by a pretty nice recursion, which makes them much more efficient than if they had to produce every ATP molecule right from scratch.
It is also interesting to note the impressive power that mitochondria work with. There are roughly a billion ATP molecules in the average cell, and each one is recycled about three times a minute. And although the human body is made up of roughly a hundred billion cells, the average person has roughly 50 g of ATP in the entire body at any one time.
(At maximum ATP demand, each mitochondrial ATP cycle can produce up to 600 ATP molecules per second. In practice, this means that if we consume two and a half thousand calories in one day, our mitochondria will recycle and reuse the same 50 grams of ATP so many times that it would be equivalent to creating roughly two hundred kilograms of ATP.)
But mitochondria have many other capabilities. They are responsible for the transmission of signals between cells (which can be interrupted by light during sleep, for example), and they participate in the cycle of cell formation and cell death. In addition, mitochondria can change their shape and size, which can be both beneficial and harmful (more on this in the next chapter), and some mitochondrial functions are unique to a particular cell type — for example, only the mitochondria in liver cells contain the enzyme needed to detoxify ammonia, a waste product produced in the liver when proteins are broken down. Different parts of the body also use ATP from mitochondria for their specific functions. For example, the heart uses the energy of mitochondria to pump blood to the brain and the rest of the body, while the brain uses their energy for thinking, learning, memory and decision making. Of course, the higher number of mitochondria in brain cells requires a lot of oxygen for ATP production, so if the mitochondria in the heart do not produce their energy efficiently enough, the brain will suffer from a lack of energy (at least, noticeably sooner than the rest of the body).
If you have less energy than you actually need, the first cells at risk will be those that are literally packed with mitochondria (such as the brain, heart, or retina).
I myself experienced a condition where my brain, due to obsessive-compulsive hyperactivity between the orbitofrontal cortex and the basal ganglia (and other related psychological ills), was massively consuming mitochondrial energy, causing me to lose weight, feel exhausted and dysfunctional. And most importantly, I was very unpleasantly depressed about it.
My disheartening experience with depression ultimately inspired me to biohack and make a general lifestyle change to support my mitochondria. There are many ways to support your oxygen-hacking bacteria, but this article is primarily about blue-light-blocking glasses, so I’ll allow myself to stay on that topic. So first, let’s take a look at how that blue light is actually making us sick at the mitochondrial level.
Dehydrated mitochondria
Blue light not only dehydrates us, but more importantly it dehydrates our mitochondria. Mitochondria, like everything living on Earth, needs water. When a mitochondria becomes dehydrated, it can no longer do what it is supposed to do. A formerly alive and functional oxygen-hacking bacterium becomes a frustrated “dead” bacterium.
This is because chronic (i.e. constant/long lasting) artificial blue light contains excessive photoelectric energy which, if delivered without adequate UV and IR color, will slow down our electron transport cycle and thus reduce water production in the mitochondria.
If this light is applied in excessive amounts, the mitochondria loses control and its electron transport cycle becomes chronically slowed and dysfunctional, resulting in no water being produced at the fourth complex of the respiratory chain and the cell becoming dehydrated.
And how do we imagine this in a way that is easy to understand?
Well, I’m sure you’ve watered your garden with a garden hose at some point. If you wanted to get the water as far as possible, you probably had to put your finger on the end (at the opening), which increased the pressure and then the water sprayed faster. Simple physics, isn’t it?
And exactly the same thing happens to our mitochondria.
People who hose their garden really honestly know that the hose will wear out over time, so a simple solution is to put duct tape over the joint where the water is leaking (i.e. where the hose is broken). But the question is, at what point does the hose break? The answer may be paradoxical to some, but it is precisely where the water goes very slowly. Not where the water is flowing fast.
So if the hose is narrower at one end and water is flowing fast there, the water is putting low pressure on the hose wall at that point. Conversely, where the water is flowing weakly, the water is exerting more pressure there, and that is where the hose is likely to burst over time.
And if you still can’t imagine it, think about it with your common sense.
If you sprint, will you have time to push? Nope. But if you walk or stand still, then you can push. Thus, where the water flow is slow, there is more pressure and the hose will break at that point before where the water is flowing fast.
When the flow of electrons on the inner membrane of the mitochondria is smooth and fast, not only is the membrane under greater stability, but it has no reason to “expand”. However, if the flow slows down, the mitochondria is forced to expand.
Its flow and particle movement affects its shape. If the mitochondrion becomes larger and stretched, the respiratory complexes are no longer close together and electrons cannot flow well. This means less water is produced. So the mitochondria dehydrate and at the same time make less deuterium-depleted water. And it also means that more deuterium will accumulate at the bottom and the whole mitochondria loses quanta of energy and becomes inefficient.
How to protect your mitochondria from dehydration?
While I want to avoid the rhetoric of the over-motivated life-coach, I will take this opportunity to mention a few simple ways to protect the mitochondria from dehydration that I implement into my life on a regular basis. And I would also venture to say that I am certainly not the only one on whom these methods of protection work.
Protect your skin from poor quality light, not from sunlight
Our skin is sensitive to light and absorbs poor quality light sources just as our eyes do. So for nighttime RegioJet trips (filled with a really extra-large dose of toxic blue light), I take something long-sleeved. A head covering (which ideally covers at least some part of my face) can protect me from the nasty blue light quite effectively, but of course you can help yourself to culture in its absence (whether you prefer a hat, cap or wig I’ll leave up to you).
However, as much natural sunlight as possible, unfiltered by glass or sunscreen, should reach our skin. That’s why I wear as little of it as possible when I’m out in healthy light.
Of course, it’s not advisable to get stuck in some attempt at perfection (no, a burqa really isn’t always the best solution to the night train). Just be careful not to stress your mitochondria in toxic office and traffic environments and cover up instead. Conversely, sunshine is your friend.
Optimise your technology
Blue light emission can be reduced quite well on screens that we have (at least some) control over. A very popular choice is e.g. the f.lux software, or Redshift (if you are on Linux and like the simpler command line mode).
As far as mobile devices are concerned, you obviously have an extremely wide choice when it comes to blue light blocking apps. Personally, I’ve used Twilight, but with my current GrapheneOS I’m perfectly satisfied with the default “Night Light” feature :)))
For Linux guys with Redhift, let me add one small reminder — if you install Redhsift, you can expect that it won’t work for you right away. You need to set it up in “Application Autostart” beforehand according to your current coordinates and Kelvin, between which your software will move during the day. For example, this is what the Redshift setup looks like with coordinates in Bratislava (-l) at 6450-1950 Kelvin (-t).
redshift-gtk -l 48.148598:17.107748 -t 6450:1950Supplement with healthy (UV!) light
To compensate for the nasty blue light, which you can only avoid in parts of North Korea and Russia today, it’s a good idea to let a healthy light work on you in a targeted way. And the best is, of course, sunshine. That’s why as part of my morning rituals I go outside, ideally barefoot. Not only does this promote vitamin D production, but my mitochondria get an extra light signal that makes them work better. And that’s only because sunlight contains the full light spectrum (from infrared to ultraviolet) that my body expects in the morning.
In addition, during the winter months, I sometimes used to treat my mitochondria in my retina to a little UV light by looking into the sun for about 30 seconds in the morning. And my ultra vices included using a UV tortoise lamp instead of a traditional nightstand lamp.
Yes, UV light (to the eyes) is harmful if there’s a lot of it. But UV light (to the eyes) is also harmful if there’s not enough of it.
There is even a study that proves that UV light received correlates with higher dopamine levels in the brain. The study specifically addresses people’s addiction to UV exposure, or the horrible drug with the terrifying name “sunbathing,” in the name of “dopamine reward”.
As ridiculous as it may sound, this study may be a nice example of how, with all the variables, we shouldn’t forget the complexity of the world and be guided primarily by our own perceptions. In all things, one must seek an individual path and find out what a given variable does specifically to me. It’s a bit silly for someone to pick up on one variable and make some health opinions based on it in civilization (I discussed this more in my previous series “Medicine Under the Guise of Dietary Narrative”), but it’s also true with UV radiation. The human body simply cannot function without UV radiation.
Sleep in the dark
The darker the night, the better. If you’re at home, turn off all electronics, turn off all lights, and use effective blackout curtains. Of course, looping in perfection isn’t necessary in this case either (otherwise you wouldn’t be able to sleep anywhere other than at home). Your goal should be to reduce the shitty light right where you currently are, while being especially careful of shitty light at the times of day when it can cause the most damage. If you spend all day under a disgusting blue light, it won’t kill you, but it will make you tired and more likely to have a sweet tooth. According to a Harvard study cited by Dave Asprey in his book Brain Strong, people with disrupted circadian rhythms have higher blood sugar and lower levels of leptin, a hormone that helps induce a feeling of satiety.
Fortunately, there are ways to protect your eyes and mitochondria from harmful light at work, stores, RegioJets, and practically everywhere.
Wear glasses!
Exposure to blue light for six and a half hours a day (which applies to most office-rat jobs) suppresses melatonin production for three hours, which is twice as long as green light. And it’s especially a bummer when you’re in an environment with poor-quality light in the two hours before you go to bed.
Which is why I wear yellow glasses at night.
Yellow glasses are a cool thing, but you have to be careful that they’re not just yellow to look at. For my nightly pilgrimages through blue light screens I use what are called Biohacker’s Evening Glasses, or Crystal Blue Light Blocking Glasses, but there are plenty of trusted companies whose eyewear covers a pretty decent portion of the spectrum affecting sleep rhythms. Biohacker’s Evening Glasses and Crystal Blue Light Blocking Glasses work well though – when I look into the blue LED with them, I see darkness instead of blue light :))
Most importantly, on the nights I put them on, Oura measures my sleep better.